Abstract
Introduction: The global JULIET trial (NCT02445248) evaluates the efficacy and safety of CTL019, a single infusion of genetically modified autologous chimeric antigen receptor-expressing T cells targeting CD19+ cells, in adult r/r DLBCL pts. Primary analyses showed a best overall response rate of 53%, which included patients with 40% complete response (CR) and 14% partial response (PR). A serious adverse event rate of 51% was observed within 8 weeks of infusion, decreasing to 23% after 8 weeks. This analysis evaluates patient reported QoL assessed before and after CTL019 infusion.
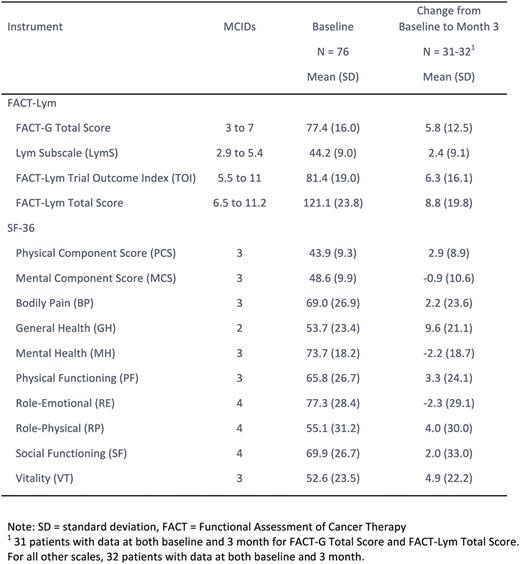
Methods: Infused pts were ≥ 18 years with chemo refractory DLBCL after receiving ≥ 2 lines of therapy and either failed autologous hematopoietic stem cell transplant (ASCT) or were otherwise ineligible or nonconsenting for ASCT. At baseline and month 3, pts completed the Short Form 36 Health Survey version 2 (SF-36) and the Functional Assessment of Cancer Therapy - Lymphoma (FACT-Lym), which includes the FACT-General (FACT-G) and the Lymphoma subscale (LymS). Both instruments are validated measures of QoL, and higher scores indicate better QoL. For the SF-36, minimally clinically important differences (MCIDs) were estimated to be 3 for the physical component score (PCS), mental component score (MCS), and vitality subscale (VT), 4 for the role-emotional (RE), role-physical (RP), and social functioning (SF) subscales, and 2 for the general health (GH) subscale. MCIDs were estimated to range from 2.9 to 5.4 for the FACT-LymS, 5.5 to 11 for the FACT-Lym trial outcome index (TOI), 6.5 to 11.2 for the FACT-Lym total score, and 3 to 7 for the FACT-G.
Results: 99 of 147 enrolled pts were infused before data cutoff; 95% had previously received 2 or more chemotherapy treatments and 48% had relapsed after SCT. 81 infused pts were followed ≥ 3 months prior to data cutoff or discontinued earlier, and were eligible for this analysis. QoL instruments were completed by 76 (94%) at baseline and 34 (42%) pts at month 3, respectively. Among 34 pts who reported PRO at 3 months, 29 pts were CR or PR, 4 pts had stable or progressive disease, and 1 pt had an unknown response status. Mean baseline scores for the FACT-LymS, FACT-Lym TOI, FACT-Lym total score, and FACT-G were 44.2, 81.4, 121.2, and 77.4, respectively, with mean change scores at month 3 of 2.4, 6.3, 8.8, and 5.8, respectively, suggesting clinically meaningful improvement in FACT-Lym, FACT-TOI and FACT-G. For the SF-36, mean baseline scores were 43.9 and 48.6 for the PCS and MCS, respectively, and ranged from 52.6 (VT) to 77.3 (RE) in the subscales. Clinically meaningful improvements in QoL were observed in the GH and VT subscales with change scores at month 3 of 9.6 and 4.9, respectively. Although point estimates exhibited wide variability, mean change scores in the PF and RP were 3.3 and 4.0, respectively, suggesting clinically meaningful improvement at month 3.
Conclusions: Clinically meaningful improvements in QoL were observed at month 3 in the FACT-Lym TOI, FACT-G and in the SF-36 GH and VT subscales and were suggested based on mean change score point estimates in the SF-36 PF, and RP. The majority of the remaining scores and subscales trended in the direction of improvement; although, MCIDs were not reached. These results suggest the intervention may improve QoL among responders after this one-time immunocellular therapy beyond the period of acute toxicities despite more than half of pts reporting SAE within 8 weeks of infusion.
Maziarz: Novartis Pharmaceuticals Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Consultancy, Honoraria; Juno Therapeutics: Honoraria; Kite Therapeutics: Honoraria; Athersys, Inc: Patents & Royalties. Tam: Roche: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; Janssen Cilag: Honoraria, Research Funding. Borchmann: Novartis Pharmaceuticals Corporation: Honoraria. Jaeger: Novartis Pharmaceuticals Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Waller: AMGEN: Consultancy; Helocyte: Consultancy; PRA: Consultancy; National Institutes of Health: Research Funding; Celldex: Consultancy; Chimerix: Equity Ownership; Coulter Foundation: Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Honoraria, Research Funding; Cerus: Equity Ownership; Katz Foundation: Research Funding; Cambium Medical Technologies: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties. Jaglowski: Novartis Pharmaceuticals Corporation: Consultancy, Research Funding; Kite Pharma: Consultancy, Research Funding; Unum Therapeutics: Research Funding; Pharmacyclics Inc: Research Funding. Andreadis: Seattle Genetics: Honoraria; Amgen: Research Funding; Gilead Sciences: Honoraria; Genentech Inc.: Employment, Equity Ownership, Honoraria; Novartis Pharmaceuticals Corporation: Honoraria, Research Funding; Pharmacyclics: Research Funding; Astellas: Honoraria; Cellerant Therapeutics: Research Funding; Incyte Pharmaceuticals: Research Funding. Foley: Novartis Pharmaceuticals Corporation: Consultancy. Fleury: Novartis Pharmaceuticals Corporation: Consultancy; Merck: Consultancy; Janssen: Consultancy; Roche: Consultancy; Seattle Genetics: Consultancy; Gilead: Consultancy; Amgen: Consultancy; Lundbeck: Consultancy. Mielke: MSD: Consultancy, Other: Travel grants; Gilead: Other: Travel grants; Cellex GmbH: Other: Travel grants, Speakers Bureau; Celgene: Other: Travel grants, Speakers Bureau; Novartis: Consultancy; KIADIS Pharma: Other: Travel grants; Jazz Pharma: Speakers Bureau; DGHO: Other: Travel support; ISCT: Other: Travel support. Westin: Kite Pharma: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Apotex: Membership on an entity's Board of Directors or advisory committees; Novartis Pharmaceuticals Corporation: Membership on an entity's Board of Directors or advisory committees. Bachanova: Novartis Pharmaceuticals Corporation: Membership on an entity's Board of Directors or advisory committees, Research Funding; Zymogen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Juno: Membership on an entity's Board of Directors or advisory committees; Oxis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle-Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees. Holte: Oslo University Hospital: Employment; Novartis Pharmaceuticals Corporation: Membership on an entity's Board of Directors or advisory committees; Nordic Nanovector: Consultancy. Anak: Novartis Pharma AG: Employment. Bubuteishvili Pacaud: Novartis Pharma AG: Employment. Guenther: Novartis Pharma AG: Employment. Zhang: Novartis Pharmaceuticals Corporation: Employment. Rasouliyan: Novartis Pharmaceuticals Corporation: Other: Lawrence Rasouliyan is an employee of RTI Health Solutions, a company that receives funding from Novartis to provide consulting services.. Tai: Novartis Pharmaceuticals Corporation: Employment. Salles: Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Morphosys: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Honoraria; MSD: Consultancy, Honoraria; Kite: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Schuster: Novartis Pharmaceuticals Corporation: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Janssen R&D: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; Seattle Genetics: Consultancy; Nordic Nanovector: Consultancy; Merck: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal